About Alcon

Alcon is the global leader in eye care, dedicated to helping people see brilliantly. With an over 75-year heritage, we are the largest eye care device company in the world, with complementary businesses in Surgical and Vision Care. Being a truly global company, we work in over 50 countries and serve patients in more than 140 countries. We have a long history of industry firsts, and each year we commit a substantial amount in Research and Development to meet customer needs and patient demands.
-

1945 Two pharmacists, Robert Alexander and William Conner, open a small pharmacy in Fort Worth, Texas, combining the first syllables of their last names to call it Alcon -

1947 Alcon Laboratories, Inc. is incorporated. The company begins manufacturing specialty pharmaceutical products -
1950 Alcon introduces its first two ophthalmic products: Ophthalzin™ for minor eye infections and Zincfrin® for red, itchy eyes -

1953 While on a sales call in West Texas, Robert Alexander and a local physician develop the DROPTAINER® eye drop dispensing bottle, the standard in eye care for years -
1962 The Alcon Trust is established, and continues today through the Alcon Foundation -
1964 Alcon establishes its charitable, in-kind donation programs: Medical Missions and U.S. Patient Assistance Programs 1969 Alcon’s surgical division is formed with seven associates -
1971 With sales of $31 million, Alcon goes public, with a listing on the New York Stock Exchange -
1989 Launch of NewVue (Focus), Alcon’s first soft contact lens exclusively for periodic replacement -
1994 The US FDA approves Alcon’s AcrySof® IQ intraocular lens (IOL), representing the first time a material had been developed specifically for an IOL. As of 2022, more than 135 million AcrySof IQ IOLs have been implanted globally -
1996 CIBA VISION launches FOCUS® DAILIES® one-day disposable contact lenses, the first lens manufactured on its proprietary LightStream™ manufacturing platform 1998 AIR OPTIX® NIGHT & DAY® launches, the first silicone hydrogel contact lens for up to 30 nights of continuous wear -

2000 Alcon introduces OPTI-FREE® EXPRESS® solution for cleaning and disinfecting soft contact lenses -
2001 AOSEPT® (CLEAR CARE® in North America) launches as the first one-bottle, peroxide lens care solution without added preservatives 2003 Alcon launches SYSTANE® eye drops for the treatment of dry eye symptoms. The SYSTANE family is now the #1 doctor recommended global artificial tear -
2004 CIBA VISION launches O2OPTIX™ (later re-branded as AIR OPTIX®), a high oxygen transmissibility, breathable monthly replacement contact lens -
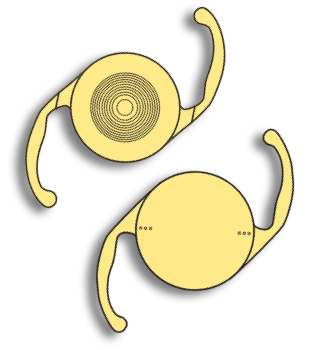
2005 Alcon launches AcrySof® IQ ReSTOR® IOL and becomes a global leader in multifocal IOLs. With portfolio expansions, including multifocal toric IOLs, Alcon introduces IOLs designed to address both astigmatism and presbyopia 2007 Alcon expands its product portfolio for LASIK surgery through the acquisition of WaveLight AG -

2008 Alcon introduces the CONSTELLATION® Vision System for vitreoretinal surgery -
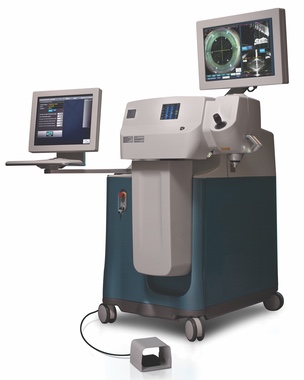
2010 Alcon expands its cataract surgical portfolio with the acquisition of LenSx Lasers, Inc.
Today, the LenSx® Laser is the global leader in cataract femtosecond laser technology -

2011 Alcon launches the world’s first-and-only water gradient daily disposable contact lens, DAILIES TOTAL1® -
2011 Alcon launches OPTI FREE® PureMoist® Multi-Purpose Disinfecting Solution with HydraGlyde® Moisture Matrix for outstanding lens surface wettability 2011 The merger of Alcon and CIBA VISION unites their strengths into one Alcon business, which today is the global leader in eye care -
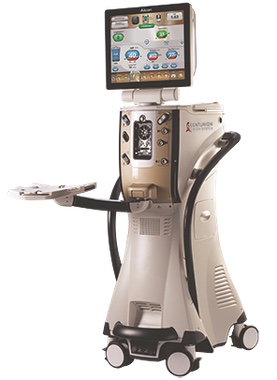
2013 Alcon launches its most advanced phacoemulsification technology platform, the CENTURION® Vision System -
2014 Alcon introduces AIR OPTIX® COLORS contact lenses, a monthly replacement color- enhancing lens with silicone hydrogel technology, now available in nine natural-looking colors -
2014 Alcon acquires the developer of the Optiwave Refractive Analysis (ORA) SYSTEM®, the first commercialized intra-operative aberrometry system for cataract surgery, which has now been used in more than two million cases worldwide -

2015 The first Alcon Experience Center (AEC), an eye health education and training center, opens. Today, there are ten AECs around the world and more than 15,000 Eye Care Professionals receive training each year -
2015 Alcon innovates in the IOL field by launching the AcrySof® IQ PanOptix® Trifocal IOL, which offers cataract patients the possibility of 20/20 vision; expands to include a toric version in 2017 -

2015 Alcon launches next generation hydrogen peroxide lens care solution, AOSEPT® PLUS with HydraGlyde® Solution (CLEAR CARE® PLUS in North America) -
2016 The NGENUITY® 3D Visualization System brings a novel heads-up display to the surgical experience, improving ergonomics for ophthalmologists -
2016 DAILIES TOTAL1® Multifocal contact lenses launch, the first-and-only water gradient contact lenses for people with presbyopia -
2018 Alcon launches SYSTANE® Complete lubricant eye drops, the first to provide relief for all major types of dry eye 2019 Alcon’s retinal portfolio expands with the launch of the HYPERVIT® Dual Blade Vitrectomy Probe -

2019 Alcon becomes an independent, publicly-traded company, after spinning off from Novartis. Alcon offers the widest array of contact lenses and surgical and ocular health products in the industry -
2019 The introduction of PRECISION1® silicone hydrogel daily disposable contact lenses offers lasting visual performance and comfort at a mainstream price -
2019 Alcon launches the LuxOR Revalia™ Ophthalmic Microscope which is designed for both anterior and posterior procedures, for every type of ophthalmic surgery -

2020 Pataday® Once Daily Relief launches in the US as the first once daily allergy itch relief drop to be available without a prescription 2020 Alcon’s retinal portfolio expands with the initial launch of the FINESSE REFLEX™ Handle for enhanced stability and control during vitreoretinal surgery -
2020 Introduction of the ARGOS® Biometer with Image Guidance provides a more efficient workflow and improved accuracy for surgeons -
2020 Alcon launches AcrySof® IQ Vivity® IOL, a first of its kind wavefront-shaping presbyopia-correcting extended depth of focus IOL with excellent vision and a monofocal-like visual disturbance profile -
2021 Alcon acquires US commercialization rights for Simbrinza®, expanding its glaucoma presence 2021 Launch of TOTAL30® contact lenses, the first-and-only monthly replacement, water gradient lens that feels like nothing, even on day 30 -
2021 Alcon introduces the first application under the SMART Solutions platform, SMARTCataract, which is designed to drive efficiency and enhanced accuracy from the clinic to the OR -

2022 Alcon completes the acquisition of Ivantis and brings the Hydrus® Microstent, a minimally invasive glaucoma surgery (MIGS) device, into the Surgical portfolio -

2022 Launch of SYSTANE® COMPLETE Preservative-Free Lubricant Eye Drops in an easy-to-use multi-dose bottle. It joins SYSTANE® ULTRA Preservative-Free and SYSTANE® HYDRATION Preservative-Free for a full portfolio of dry eye relief products -
2022 Alcon announces the roll out of the CLAREON® family of IOLs, featuring the novel CLAREON material that can deliver consistent visual outcomes -

2022 Alcon introduces Fidelis Virtual Reality (VR) Ophthalmic Surgical Simulator, a portable VR tool for cataract surgeons-in-training
Our Business Focus
Our leadership is grounded in cutting-edge innovation and breakthrough technology, transforming the way we treat eye diseases and eye conditions. We have the widest array of eye care offerings in the industry with products organized into two business: Surgical and Vision Care.
Surgical

Surgical
We offer the industry’s most complete line of ophthalmic surgical products, enabling surgeons to achieve the best results for their patients. Our surgical portfolio includes technologies and devices for cataract, retinal, refractive surgery, as well as advanced technology intraocular lenses (ATIOLs) to treat cataracts and refractive errors, like presbyopia and astigmatism. We also provide advanced viscoelastics, surgical solutions, surgical packs, and other disposable products for cataract and vitreoretinal surgery.

Vision Care

Vision Care
We are one of the largest manufacturers of contact lenses and lens care products. Our Vision Care portfolio offers a broad range of daily disposable, reusable and color-enhancing contact lenses and a comprehensive portfolio of ocular health products. These include products for dry eye, contact lens care and ocular allergies, as well as ocular vitamins and redness relievers.

